トップページ > 診療科・部門 > センター > 国際リンパ浮腫センター > Supermicrosurgery International Lymphedema Center (SILC)
Supermicrosurgery International Lymphedema Center (SILC)
(Supermicrosurgical Treatments for Lymphedema)
Lymphedema
Lymphedema is an edematous disease caused by abnormal lymph circulation, and largely classified into primary lymphedema and secondary lymphedema. Secondary lymphedema is caused by lymph flow obstruction resulting from cancer treatments (lymph node dissection and/or radiotherapy), trauma, infection, etc. Primary lymphedema develops progressive edema in which causative mechanism is unknown. In tropical areas, lymphedema secondary to filarial infection is the most common, whereas cancer treatments are the most common cause of lymphedema in developed countries.
After cancer treatments including lymphadenectomy and/or radiotherapy, lymph flows are obstructed in the treated region; lymph flows are obstructed in the axilla after breast cancer treatments, and in the pelvis after treatments for pelvic cancer such as uterine cancer, ovarian cancer, etc. Lymph flow obstruction leads to congestion of lymph and dilatation of lymphatic vessels distal to the obstruction site. First, lymphedema manifests as “pitting-edema”, and then as “non-pitting edema” with progression of lymphedema over time. Since lymph contains immune cells, congestion of lymph leads to local immune deficiency, causing frequent cellulitis. With long-standing lymphedema with frequent inflammatory episodes, lymphedema would result in elephantiasis, and be complicated with malignant tumor such as angiosarcoma (called “Stewart-Treves syndrome”).
It is essential to improve abnormal lymph circulation “the cause of lymphedema” by early interventions; otherwise lymphedema progresses over time without
improvement.
Diagnosis of Lymphedema
Diagnosis of lymphedema starts from excluding other edematous diseases such as heart, kidney, liver, endocrine, malignant, and venous diseases (ruled out by internists before visiting lymphedema clinic). Then, lymph imaging studies are performed to evaluate lymph circulation; when abnormal lymph circulation is determined, lymphedema is diagnosed.
Lymph imaging studies include lymphoscintigraphy (SPECT-CT), MR lymphography, and ICG lymphography. Based on characteristics of lymphedema, several studies are combined to evaluate the disease comprehensively. Among them, ICG lymphography is especially useful for lymphedema evaluation and management. ICG lymphography allows pathophysiological severity staging useful for prediction of lymphedema progression and for considering indication of lymphedema treatments including lymphatic supermicrosurgeries.
Treatments for Lymphedema
Compression garments and bandage are mainstays of lymphedema treatments. Once lymphedema develops, it progresses over time without cure regardless of subjective symptoms; stopping compression always results in progressive deterioration of lymphedema. Since compression is only an anti-symptomatic treatment (not curative one), life-long compression is necessary for patients with lymphedema. Manual lymph drainage (MLD), massage therapy specialized for lymphedema treatment performed by a trained therapist, is applied to treat lymphedema. MLD is useful to aggressively reduce lymphedematous volume when performed continuously (usually under hospitalization); MLD is hardly effective when performed under an outpatient setting. “lymph massage” other than MLD should not be performed, because lymphatics may be damaged and lymphedema may go worse.
Surgical treatments are performed for progressive lymphedema refractory to conservative treatments. In the past, debulking surgeries and “classical lymphaticovenous anastomosis” (lympahtics are inserted into a vein) have been performed, but abandoned because of their invasiveness and unsatisfactory results. With advancement of surgical techniques and instruments, “supermicrosurgery” (sophisticated microsurgical techniques allowing anastomosis of vessels with diameter of 0.5 mm or smaller) has been developed. Using supermcirosurgical skills, “true lymphatic vessel anastomosis” (in an intima-to-intima coaptation manner) becomes possible, which leads to development of supermicrosurgical “lymphaticovenular anastomosis (LVA)”. Currently, 3 surgical treatments are applied in lymphedema surgery; LVA, vascularized lymph node transfer (LNT), and liposuction (LS). Based on ICG lymphography staging (DB stage), appropriate treatments are selected to obtain optimal results.
Lymph flow evaluation using ICG lymphography
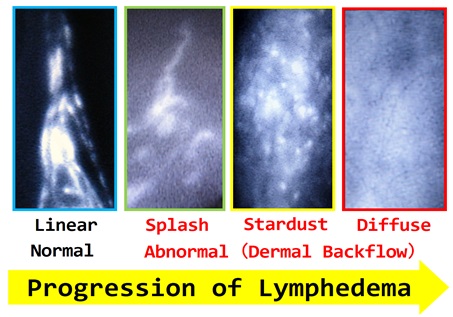
(With progression of lymphedema, ICG lymphography findings change from Linear, to Splash, to Stardust, and finally to Diffuse pattern)
- Yamamoto T, et al.
Characteristic indocyanine green lymphography findings in lower extremity lymphedema: the generation of a novel lymphedema severity staging system using dermal backflow patterns.
Plast Reconstr Surg. 2011;127(5):1979-86.
ICG lymphography staging and lymphedema management
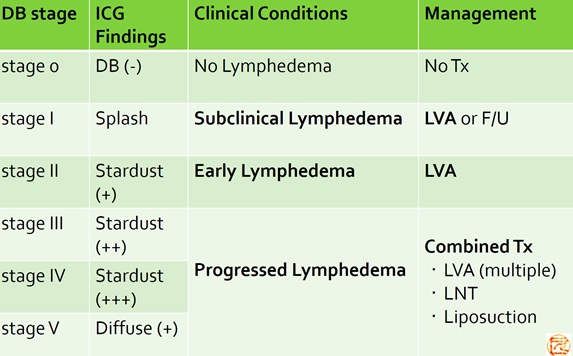
(Therapeutic strategy based on pathophysiological staging)
- Yamamoto T, et al.
The earliest finding of indocyanine green (ICG) lymphography in asymptomatic limbs of lower extremity lymphedema patients secondary to cancer treatment: the modified dermal backflow (DB) stage and concept of subclinical lymphedema.
Plast Reconstr Surg. 2011;128(4):314e-21e. - Yamamoto T, et al.
Subclinical lymphedema: understanding is the clue to decision making.
Plast Reconstr Surg. 2013;132(3):472e3e.
Supermicrosurgical Lymphaticovenular Anastomosis (LVA)
Lymph originally flows into venous circulation at the venous angle (neck), and fluid is discharged as urine through the kidney. Lymphedema is caused by lymph flow obstruction distal to the venous angle (before flowing into venous circulation) by cancer surgery, etc.. In LVA surgery, congested lymph flow is diverted into venous circulation distal to obstruction site by creating lymphovenous shunt(s); in the lower extremity for uterine cancer treatment-related lymphedema, in the upper extremity for breast cancer treatment-related lymphedema, etc.. Unlike “classical lymphaticovenous anastomosis”, lymphatic vessels are anastomosed to nearby small veins (venules) in an intima-to-intima coaptation manner by supermicrosurgery; both lymphatic vessel and recipient vein are approximately 0.5 mm, and a 50-65 micron needle (11-0 nylon or 12-0 nylon) is used for supermicrosurgical anastomosis. Since the endothelium (intima) is covered at an anastomosis site, anastomosis site thrombosis can be prevented even when venous reflux occurs in LVA; on the other hand, anastomosis thrombosis is inevitable in classical lymphaticovenous anastomosis, because tissue other than intima is exposed in vessel lumen.
LVA is the least invasive surgical treatment for lymphedema; can be performed via approximately 2 cm skin incisions under local infiltration. As LVA addresses pathophysiology of lymphedema by decongesting lymph with lymphovenous shunt, LVA can be effective for compression-refractory lymphedema allowing reduction of cellulitis frequency, compression therapy, and even cure of lymphedema (volume reduction without maintenance compression therapy; only possible for subclinical/early lymphedema). Since the number of anastomoses is positively associated with volume reduction, LVA surgeons try to create as many anastomoses as possible within a given operation time (usually around 2 hours, max 4 hours). Although LVA can be effective for progressive lymphedema refractory to conservative treatments, we cannot assure good results after LVA for all patients; lymphatic vessel becomes sclerotic with progression of lymphedema, which results to ineffective lymphovenous bypass.
Highly sophisticated supermicrosurgical techniques and enough experience are required to do LVA surgery. In Department of Plastic and Reconstructive Surgery at NCGM, a well-experienced supermicrosurgeon, who have anastomosed more than 6,000 lymphatic vessels (see staff information and below), operates LVA with assistant operators (Japanese and non-Japanese doctors join LVA surgery).)
Consultation
Lymphedema patients with definitive diagnosis of lymphedema based on lymphoscintigraphy, SPECT/CT, MR lymphography, or ICG lymphography can be treated at NCGM. Especially for patients with primary lymphedema, other edematous diseases must be ruled out by thorough evaluations. Since LVA works only with postoperative compression, LVA surgery is indicated only for patients compliant to strict compression therapy (pre- and post-operatively). Patients with systemic comorbidities or allergic history cannot undergo LVA surgery at NCGM. For more precise information, please contact to International Health Care Center (support@hosp.ncgm.go.jp).
Publication of scientific articles and textbooks
- Yamamoto T, et al.
Lambda-shaped anastomosis with intravascular stenting method for safe and effective lymphaticovenular anastomosis.
Plast Reconstr Surg. 2011;127(5):1987-92. - Yamamoto T, et al.
The earliest finding of indocyanine green (ICG) lymphography in asymptomatic limbs of lower extremity lymphedema patients secondary to cancer treatment: the modified dermal backflow (DB) stage and concept of subclinical lymphedema.
Plast Reconstr Surg. 2011;128(4):314e-21e. - Yamamoto T, et al.
Upper Extremity Lymphedema (UEL) Index: A Simple Method for Severity Evaluation of Upper Extremity Lymphedema.
Ann Plast Surg. 2011 Jul 5 [Epub ahead of print] - Yamamoto T, et al.
Indocyanine green (ICG)-enhanced lymphography for upper extremity lymphedema: a novel severity staging system using dermal backflow (DB) patterns.
Plast Reconstr Surg. 2011 Jun 15. [Epub ahead of print] - Yamamoto T, et al.
Indocyanine green (ICG)-enhanced lymphography for evaluation of facial lymphoedema.
J Plast Reconstr Aesthet Surg. 2011 Jun 15 [Epub ahead of print] - Yamamoto T, et al.
Characteristic indocyanine green lymphography findings in lower extremity lymphedema: the generation of a novel lymphedema severity staging system using dermal backflow patterns.
Plast Reconstr Surg. 2011;127(5):1979-86. - Yamamoto T, et al.
Lower extremity lymphedema index: a simple method for severity evaluation of lower extremity lymphedema.
Ann Plast Surg. 2011 Mar 14 [Epub ahead of print]. - Yamamoto T, et al.
Simultaneous multi-site lymphaticovenular anastomoses for primary lower extremity and genital lymphoedema complicated with severe lymphorrhea.
J Prast Reconstr Aesthet Surg. 2011;64(6):812-5. Epub2010 Nov 17. - Yamamoto T, et al.
LEC score: A judgment tool for indication of indocyanine green lymphography.
Ann Plast Surg. 2013;70(2):227-30. - Yamamoto T, et al.
Split intravascular stents for side-to-end lymphaticovenular anastomosis.
Ann Plast Surg. 2013;71(5):538-40. - Yamamoto T, et al.
Indocyanine green velocity: Lymph transportation capacity deterioration with progression of lymphedema.
Ann Plast Surg. 2013;71(5):591-4. - Yamamoto T, et al.
Minimally invasive lymphatic supermicrosurgery (MILS): indocyanine green-guided simultaneous multi-site lymphaticovenular anastomoses via millimeter skin incisions.
Ann Plast Surg. 2012 Dec 13 [Epub ahead of print]. - Yamamoto T, et al.
A modified side-to-end lymphaticovenular anastomosis.
Microsurgery. 2013;33(2):130-3. - Yamamoto T, et al.
Sequential anastomosis for lymphatic supermicrosurgery: multiple lymphaticovenular anastomoses on one venule.
Ann Plast Surg. 2012 Dec 13 [Epub ahead of print]. - Yamamoto T, et al.
Dynamic indocyanine green lymphography for breast cancer-related arm lymphedema.
Ann Plast Surg. (in press). - Yamamoto T, et al.
Side-to-end lymphaticovenular anastomosis through temporary lymphatic expansion.
PLoS ONE. 2013;8(3):e59523. Epub 2013 Mar 25. - Yamamoto T, et al.
Indocyanine green lymphography for evaluation of genital lymphedema in secondary lower extremity lymphedema patients.
J Vasc Surg Venous Lymphat Dis. 2013 July 18 [Epub ahead of print]. - Yamamoto T, et al.
Near-infrared illumination system-integrated microscope for supermicrosurgical lymphaticovenular anastomosis.
Microsurgery. 2013 Jul 9 [Epub ahead of print]. - Yamamoto T, et al.
Subclinical lymphedema: understanding is the clue to decision making.
Plast Reconstr Surg. 2013;132(3):472e3e. - Yamamoto T, et al.
Modified lambda-shaped lymphaticovenular anastomosis with supermicrosurgical lymphoplasty technique for a cancer-related lymphedema patient.
Microsurgery. 2013 Sep 13 [Epub ahead of print]. - Yamamoto T, et al.
Colourful indocyanine green lymphography.
J Plast Reconstr Aesthet Surg. 2013 Oct 1 [Epub ahead of print]. - Yamamoto T, et al.
Neo-valvuloplasty for lympahtic supermicrosurgery.
J Plast Reconstr Aesthet Surg. 2014;67(4):587-8. - Yamamoto T, et al.
Ladder-shaped lymphaticovenular anastomosis using multiple side-to-side lymphatic anastomoses for a leg lymphedema patient.
Microsurgery. 2014;34(5):404-8. - Yamamoto T, et al.
Upward retraction for lymphaticovenular anastomosis in the deep fat layer.
Microsurgery. 2014;34(7):586-7. - Yamamoto T, et al.
Technical simplification of the supermicrosurgical side-to-end lymphaticovenular anastomosis using the parachute technique.
Microsurgery. 2015 Feb;35(2):129-34. - Yamamoto T, et al.
Splash, stardust, or diffuse pattern: differentiation of dermal backflow pattern is important in indocyanine green lymphography.
Plast Reconstr Surg. 2014;133(6):e887-8. - Yamamoto T, et al.
Navigation lymphatic supermicrosurgery for iatrogenic lymphorrhea: supermicrosurgical lymphaticolymphatic anastomosis and lymphaticovenular anastomosis under indocyanine green lymphography navigation.
J Plast Reconstr Aesthet Surg. 2014;67(11):1573-9. - Yamamoto T, et al.
Triple supermicrosurgical side-to-side lymphaticolymphatic anastomoses on a lymphatic vessel end-to-end anastomosed to a vein.
Microsurgery. 2014 Jul 15 [epub ahead of print] - Yamamoto T, et al.
Mono-canalization of adhered lymphatic vessels for lymphatic supermicrosurgery.
J Plast Reconstr Aesthet Surg. 2014;67(11):e291-2. 2014 Jul 31 [epub ahead of print] - Yamamoto T, et al.
Lymph preserving lipectomy under indocyanine green lymphography navigation.
J Plast Reconstr Aesthet Surg. 2015;68(1):136-7. - Yamamoto T, et al.
Supermicrosugical anastomosis of superficial lymphatic vessel to deep lymphatic vessel for a patient with cellulitis-induced chronic localized leg lymphedema.
Microsurgery. 2015;35(1):68-71. 2014 Sep 8 [epub ahead of print] - Yamamoto T, et al.
Establishment of supermicrosurgical lymphaticovenular anastomosis model in rat.
Microsurgery. 2014 Oct 3 [Epub ahead of print] - Yamamoto T, et al.
Multiple-In-One concept for lymphatic supermicrosurgery.
Microsurgery. 2014 Oct 28 [epub ahead of print] - Yamamoto T, et al.
Efferent lymphatic vessel anastomosis (ELVA): supermicrosurgical efferent lymphatic vessel-to-venous anastomosis for the prophylactic treatment of subclinical lymphedema.
Ann Plast Surg. 2014 Nov 1 [Epub ahead of print] - Yamamoto T, et al.
Indocyanine green lymphography findings in primary leg lymphedema.
Eur J Vasc Endovasc Surg. 2015;49:95-102. 2014 Dec 3 [Epub ahead of print] - Yamamoto T, et al.
Supermicrosurgical deep lymphatic vessel-to-venous anastomosis for a breast cancer-related arm lymphedema with severe sclerosis of superficial lymphatic vessels.
Microsurgery. 2015 Jan 17 [Epub ahead of print] - Yamamoto T, et al.
Relationship between lymphedema and arteriosclerosis: higher cardio-ankle vascular index in lymphedematous limbs.
Ann Plast Surg. 2015 Feb 18 [Epub ahead of print] - Yamamoto T, et al.
Hands-free vein visualizer for selection of recipient vein with an intact valve in lymphatic supermicrosurgery.
J Plast Reconstr Aesthet Surg. 2015 Feb 14 [Epub ahead of print] - Yamamoto T, et al.
A method of continuous indirect aspiration for field clearance in lymphatic supermicrosurgery.
Microsurgery. 2015 May 19 [Epub ahead of print] - Yamamoto T, et al.
Lymphatic vessel grafting for prevention of venous reflux into a sclerotic lymphatic vessel in supermicrosurgical lymphaticovenular anastomosis.
J Plast Reconstr Aesthet Surg. 2016 Jan 7 [epub ahead of print] - Yamamoto T, et al.
Microsurgical venous-branch-plasty for approximating diameter and vessels' position in lymphatic supermicrosurgery.
J Plast Reconstr Aesthet Surg. 2016 Feb 17 [epub ahead of print] - Yamamoto T, et al.
Quadruple-component superficial circumflex iliac artery perforator (SCIP) flap: a chimeric SCIP flap for complex ankle reconstruction of an exposed artificial joint after total ankle arthroplasty.
J Plast Reconstr Aesthet Surg. 2016 Jun 23 [epub ahead of print] - Yamamoto T, et al.
Complete lymph flow reconstruction: a free vascularized lymph node true perforator flap transfer with efferent lymphaticolymphatic anastomosis.
J Plast Reconstr Aesthet Surg. 2016 Jul 2 [epub ahead of print] - Yamamoto T, et al.
Fusion lymphoplasty for diameter approximation in lymphatic supermicrosurgery using two lymphatic vessels for a larger recipient vein.
J Plast Reconstr Aesthet Surg. 2016 Jul 9 [epub ahead of print] - Yamamoto T, et al.
Time to re-consider a gold standard of lymph flow imaging: importance of reliability to detect abnormal lymphodynamics in lymphedema screening after cancer treatments.
Plast Reconstr Surg. 2016 Dec 9 [epub head of print] - Yamamoto T, et al.
Application of a multi-directional transformable retractor for lymphatic supermicrosurgery using SEKI method.
Microsurgery. 2016 Dec 23 [epub ahead of print] - Yamamoto T, et al.
Indocyanine green lymphography for lymphedema screening following breast cancer treatment.
Plast Reconstr Surg 2017 Feb 14 [Epub ahead of print] - Yamamoto T, et al.
Factors associated with lower extremity dysmorphia caused by lower extremity lymphedema.
Eur J Vasc Endovasc Surg. 2017 Apr 6 [epub head of print] - Yamamoto T, et al.
Lymphatic vessel diameter in female pelvic cancer-related lower
extremity lymphedematous limbs.
J Surg Oncol 2018 Jan 22 [epub ahead of print] - Yamamoto T, et al.
Possible optimal donor site for multiple lymph node transfers.
J Am Coll Surg 2018 Feb;226(2):202-3. - Yamamoto T, et al.
Lymph flow restoration after tissue replantation and transfer: importance of lymph axiality and possiblity of lymph flow reconstruction using free flap transfer without lymph node or supermicrosurgical lymphatic anastomosis.
Plast Reconstr Surg 2018 Jun 22 [epub ahead of print] - Yamamoto T, et al.
Optimal sites for supermicrosurgical lymphaticovenular anastomosis: an analysis of lymphatic vessel detection rates on 840 surgical fields in lower extremity lymphedema.
Plast Reconstr Surg. 2018 Sep 4 [epub ahead of print] - Yamamoto T, et al.
Targeting reflux-free veins with a vein visualizer to identify the ideal recipient vein preoperatively for optimal lymphaticovenous anastomosis in treating lymphedema.
Plast Reconstr Surg. 2018 Nov;142(5):804e-806e. - Yamamoto T.
Onco-Reconstructive Supermicrosurgery.
Eur J Surg Oncol. 2019 Jan 9 [epub ahead of print] - Yamamoto T.
Impact of lower extremity dysmorphia on lymphedema patients' quality of life.
Plast Reconstr Surg. 2019 Feb 1 [epub ahead of print] - Tsukuura R, Sakai H, Fuse Y, Yamamoto T.
Novel hands-free near-infrared fluorescence navigation and simultaneous combined imaging for elevation of vascularized lymph node flap.
J Surg Oncol 2018 Sep;118(3):588-89. - Fuse Y, Yamamoto T.
Diamond-shaped anastomosis for supermicrosurgical side-to-side lymphaticovenular anastomosis.
J Plast Reconstr Aesthet Surg 2015 Sep 5 [epub ahead of print] - Fuse Y, Yamamoto T.
Half notching method for supermicrosurgical lambda-shaped lymphaticovenular anastomsois.
J Plast Reconstr Aesthet Surg 2015 Sep 5 [epub ahead of print] - Fuse Y, Yamamoto T, Saito T, Ishiura R, Iida T.
Near-infrared fluorescent swallow test for detection of the alimentary tract anastomotic leakage.
J Plast Reconstr Aesthet Surg 2015 Oct 9 [epub ahead of print] - Fuse Y, Yoshimatsu H, Yamamoto T.
Lateral approach to the deep branch of the superficial circumflex iliac artery for harvesting a SCIP flap.
Microsurgery Feb 23 [epub ahead of print] - Fuse Y, Yamamoto T.
Intraoperative distal compression in supermicrosurgical lymphaticovenous anastomosis for lymphedema.
J Surg Oncol 2018 Jun 7 [epub ahead of print] - Sakai H, Yamamoto T, Yamamoto N, Fuse Y.
Modified fusion lymphoplasty for approximation of diameter and distance between two lymphatic vessels and a larger recipient vein.
Microsugery 2017 Sep 19 [epub ahead of print] - Sakai H, Yamamoto T, Fuse Y.
Lympahtic vessel diameter and lymphosclerosis: two different characteristics.
Lymphat Res Biol. 2018 Jun;16(3):317 - Sato Y, Fuse Y, Yamamoto T.
Indocyanine green lymphography for diagnosis of lymphedema following thigh lift surgery.
Microsurgery 2018 Jun 19 [epub ahead of print] - Yamamoto T, Iida T, Yoshimatsu H, Fuse Y, Hayashi A, Yamamoto N.
Yamamoto T restoration after tissue replantation and transfer: importance of lymph axiality and possibility of Yamamoto T reconstruction using free flap transfer without lymph node or supermicrosurgical lymphatic anastomosis.
Plast Reconstr Surg 2018 Sep;142(3):796-804 - Yoshimatsu H, Yamamoto T, Tanakura K, Fuse Y, Hayashi A.
"Powered" lymphaticovenular anastomosis for treatment of upper extremity lymphedema: deducing location of functional lymphatic vessels from pumping mavement of the underlying muscles.
Plast Reconstr Surg. 2018 Jul 20 [epub ahead of print] - Ando Y, Yamamoto T, Fuse Y.
An intraoperative 3D imaging system for better image sharing and protection of reconstructive surgeons' neck. Plast Reconstr Surg. 2018 Aug 14 [epub ahead of print] - Yamamoto T, Yamamoto N, Sakai H, Fuse Y, Yoshimatsu H, Seki Y, Kajikawa A.
Lymphedema quality of life score (LeQOLiS): A simple method for evaluation of subjective symptoms in extremity lymphedema patients.
Plast Reconstr Surg. 2018 Aug 14 [epub ahead of print] - Narushima M, Yamasoba T, Iida T, Matsumoto Y, Yamamoto T, Yoshimatsu H, Timothy S, Pafitanis G, Yamashita S, Koshima I.
Pure skin perforator flaps: the anatomical vascularity of the superthin flap.
Plast Reconstr Surg. 2018 Sep;142(3):351e-360e. - Tsukuura R, Sakai H, Fuse Y, Yamamoto T.
Novel hands-free near-infrared fluorescence navigation and simultaneous combined imaging for elevation of vascularized lymph node flap.
J Surg Oncol 2018 Sep;118(3):588-89. - Yamamoto T, Yamamoto N, Fuse Y, Narushima M, Koshima I.
Optimal sites for supermicrosurgical lymphaticovenular anastomosis: an analysis of lymphatic vessel detection rates on 840 surgical fields in lower extremity lymphedema.
Plast Reconstr Surg. 2018 Dec;142(6):924e-930e. - 72・Yoshimatsu H, Yamamoto T, Tanakura K, Fuse Y, Hayashi A. Noncontrast magnetic resonance lymphography for evaluation of lymph node transfer for secondary upper limb lymphedema. Plast Reconstr Surg 2018 Oct;142(4):601e-603e.
- Seki Y, Kajikawa A, Yamamoto T, Takeuchi T, Terashima T, Kurogi N.
The dynamic-lymphaticovenular anastomosis method for breast cancer treatment-related lymphedema: creation of functional lympahticovenular anastomoses with use of preoperative dynamic ultrasonography.
J Plast Reconstr Aesthet Surg. 2018 Sep 20 [epub ahead of print] - Yamamoto T, Yamamoto N, Sakai H, Fuse Y, Yoshimatsu H, Seki Y, Kajikawa A.
Targeting reflux-free veins with a vein visualizer to identify the ideal recipient vein preoperatively for optimal lymphaticovenous anastomosis in treating lymphedema.
Plast Reconstr Surg. 2018 Nov;142(5):804e-806e.・ - Yoshimatsu H, Steinbacher J, Meng S, Hamscha UM, Weninger WJ, Tinhofer IE, Harima M, Fuse Y, Yamamoto T, John Tzou CH.
Surgical anatomy of the supeficial circumflex iliac artery perforator flap: an anatomic study of the correlation of the superficial and the deep branches of the superficial circumflex iliac artery and evaluation of perfusion from the deep branch to the sartorius muscle and the iliac bone.
Plast Reconstr Surg. 2018 Nov 26 [epub ahead of print] - Pafitanis G, Narushima M, Yamamoto T, Miller R, Koshima I. Insights to establish early learning curve in clinical supermicrosurgery. Microsurgery. 2018 Dec 17 [epub ahead of print]
- Brahma B, Yamamoto T.
Breast cancer treatment-related lymphedema (BCRL): an overview of the literature and updates in microsurgery reconstruction.
Eur J Surg Oncol. 2019 Jan 4 [epub ahead of print] - Yamamoto T.
Onco-Reconstructive Supermicrosurgery.
Eur J Surg Oncol. 2019 Jul;45(7):1146-1151. - Ganry L, Fuse Y, Sakai H, Reiko T, Yamamoto T.
Refinement of the chicken wing supermicrosurgical training model: pre-operative indocyanine green injection highlighting vessels' visualization under 0.4mm of diameter.
Microsurgery Jan 21 [epub ahead of print] - Yamamoto T.
Impact of lower extremity dysmorphia on lymphedema patients' quality of life.
Plast Reconstr Surg. 2019 Feb 1 [epub ahead of print] - Giacalone G, Yamamoto T, Hayashi A, Belva F, Gysen M, Hayashi N, Yamamoto N, Koshima I.
Lymphatic supermicrosurgery for the treatment of recurrent lymphocele and severe lymphorrhea.
Microsurgery 2019 Feb 14 [epub ahead of print] - Hayashi A, Giacalone G, Yamamoto T, Belva F, Visconti G, Hayashi N, Handa M, Yoshimatsu H, Salgarello M. Ultra high-frequency ultrasonographic imaging with 70 MHz scanner for visualization of the lymphatic vessels. Plast Reconstr Surg Glob Open. 2019 Jan 22;7(1):e2086.
- Yoshimatsu H, Harima M, Iida T, Narushima M, Karakawa R, Nakatsukasa S, Yamamoto T, Hayashi A.
Use of the distal facial artery (angular artery) for supermicrosurgical midface reconstruction.
Plast Reconstr Surg Glob Open. 2019 Feb 5;7(2):e1978. - Giacalone G, Yamamoto T, Belva F, Hayashi A, Dori Y, Zviman MM, Gysen M, Nam HH, Jolley MA, Kato M.
The application of virtual reality for preoperative planning of lymphovenous anastomosis in a patient with a complex lymphatic malformation.
J Clin Med. 2019 Mar 15;8(3):E371. - Giacalone G, Yamamoto T, Belva F, Wets R, Hayashi A, Koshima I.
Successful treatment of breast cancer-related breast lymphedema by lymphovenous anastomosis in a male patient.
Microsurgery. 2019 Mar 19 [epub ahead of print] - Giacalone G, Yamamoto T, Hayashi A, Belva F, Seki Y.
An L-shaped retractor to facilitate the superior-edge-of-the-knee-incision-method for lympho-venous anastomosis.
Microsurgery. 2019 Mar 22 [epub ahead of print] - Giacalone G, Yamamoto T, Yamamoto N.
Piority claim: first lymphovenous anastomosis performed for breast lymphedema in 2016 and 2017.
Ann Plast Surg 2019 Apr15 [epub ahead of print] - Hosoya S, Yamamoto T.
Stump staining for clear visualization of lymphatic vessel's lumen.
J Plast Reconstr Aesthet Surg. 2019 May 17 [epub ahead of print] - Seki Y, Kajikawa A, Yamamoto T, Takeuchi T, Terashima T, Kurogi N.
Real-time indocyanine green videolymphography navigation for lymphaticovenular anastomosis.
Plast Reconstr Surg Glob Open. 2019 May 24;7(5):e2253. - Fuse Y, Prasad V, Yoshimatsu H, Yamamoto T.
"Quadrupod" grip for handling supermicrosurgical instruments.
J Reconstr Microsurg 2019 Aug 9 [epub ahead of print] - Tsuihiji K, Tsukuura R, Yamamoto T.
Absorbable plating of nasoorbitoethmoid fractures for adults.
J Craniofac Surg. 2019 Oct;30(7).2298-2298. - Yoshimatsu H, Yamamoto T, Hayashi A, Fuse Y, Karakawa R, Iida T, Narushima M, Tanakura K, Weninger WJ, Tzou CHJ.
Use of the transverse branch of the superficial circumflex iliac artery as a landmark facilitating identification and dissection of the deep branch of the superficial circumflex iliac artery for free flap pedicle: anatomical study and clinical applicatoins.
Microsurgery. 2019 Oct 8 [epub ahead of print] - Visconti G, Hayashi A, Tartaglione G, Yamamoto T, Bianchi A, Salgarello M.
Preoperative planning of lymphaticoevnular anastomosis in patients with iodine allergy: multicentric experience.
J Plast Reconstr Aesthet Surg. 2019 Nov 29 [epub ahead of print] - Yoshimatsu H, Hayashi A, Yamamoto T, Visconti G, Karakawa R, Fuse Y, Iiad Y.
Visualization of the "intradermal plexus" using ultrasonography in the dermis flap: a step beyond perforator flaps.
Plast Reconstr Surg Glob Open. 2019 Nov 14;7(11):e2411 - Yamamoto T, Yamamoto N, Kageyama T, Sakai H, Fuse Y, Tsuihiji K, Tsukuura R.
Definition of perforator flap: what does a "perforator" perforate?
Global Health & Medicine. 2019; 1(2):114-116. DOI: 10.35772/ghm.2019.01009 - Yamamoto T, Yamamoto N, Kageyama T, Sakai H, Fuse Y, Tsuihiji K, Tsukuura R.
Supermicrosurgery for oncologic reconstructions.
Global Health & Medicine. 2020; 2(1):18-23. DOI: 10.35772/ghm.2019.01019 - Arie A, Yamamoto T.
Buffalo skull-shaped supermicrosurgical lymphaticovenular anastomosis.
J Plast Reconstr Aesthet Surg. 2020 Feb 20 [published online] - Giacalone G, Yamamoto T, Belva F, Hayashi A.
Bedside 3D visualization of lymphatic vessels with a handheld multispectral optoacoustic tomography device.
J Clin Med. 2020;9(3):e815 - Yamamoto T, Yamamoto N, Kageyama T, Sakai H, Fuse Y, Tsuihiji K, Tsukuura R.
Technical pearls in lymphatic supermicrosurgery.
Global Health & Medicine. 2020; 2(1):29-32. DOI: 10.35772/ghm.2019.01010 - Yamamoto T.
Congenital Vascular Malformation- Comprehensive Review on Current Management. Part V. Contemporary Diagnosis: Imaging Modalities. Chapter 25. Indocyanine Green (ICG) Lymphography.
Springer - Yamamoto T.
ICG Fluorescence imaging and navigation surgery. Chapter 44. Comprehensive Lymphedema Evaluation using Dynamic ICG Lymphography.
Springer
etc.